A Key Function Of Cellular Respiration Is To Generate:
Learning Objectives
- The Main Function Of Cellular Respiration Is To Generate
- A Key Function Of Cellular Respiration Is To Generate Quizlet
- Advantages Of Cellular Respiration
- Location Of Cellular Respiration
- Function Of Fermentation
- A Key Function Of Cellular Respiration Is To Generate: Pdf
- Compare and contrast the electron transport system location and function in a prokaryotic cell and a eukaryotic cell
- Compare and contrast the differences between substrate-level and oxidative phosphorylation
- Explain the relationship between chemiosmosis and proton motive force
- Describe the function and location of ATP synthase in a prokaryotic versus eukaryotic cell
- Compare and contrast aerobic and anaerobic respiration
We have just discussed two pathways in glucose catabolism—glycolysis and the Krebs cycle—that generate ATP by substrate-level phosphorylation. Most ATP, however, is generated during a separate process called oxidative phosphorylation, which occurs during cellular respiration. Cellular respiration begins when electrons are transferred from NADH and FADH2—made in glycolysis, the transition reaction, and the Krebs cycle—through a series of chemical reactions to a final inorganic electron acceptor (either oxygen in aerobic respiration or non-oxygen inorganic molecules in anaerobic respiration). These electron transfers take place on the inner part of the cell membrane of prokaryotic cells or in specialized protein complexes in the inner membrane of the mitochondria of eukaryotic cells. The energy of the electrons is harvested to generate an electrochemical gradient across the membrane, which is used to make ATP by oxidative phosphorylation.
- The variation in the amount of ATP produced by aerobic respiration reflects the fact that plants cell sometimes squeeze 38 ATP from one glucose molecule, while animal cells generate 36 ATP per glucose molecule. This ATP comes from combining free phosphate molecules (P).
- When we talk related with Chapter 9 Cellular Respiration Worksheet, scroll the page to see various similar photos to inform you more. Cellular respiration worksheet answer key, function of the cell: welcome to modern biology and respiratory system worksheet answer key are three of main things we will present to you based on the gallery title.
Electron Transport System
The electron transport system (ETS) is the last component involved in the process of cellular respiration; it comprises a series of membrane-associated protein complexes and associated mobile accessory electron carriers. Electron transport is a series of chemical reactions that resembles a bucket brigade in that electrons from NADH and FADH2 are passed rapidly from one ETS electron carrier to the next. These carriers can pass electrons along in the ETS because of their redox potential. For a protein or chemical to accept electrons, it must have a more positive redox potential than the electron donor. Therefore, electrons move from electron carriers with more negative redox potential to those with more positive redox potential. The four major classes of electron carriers involved in both eukaryotic and prokaryotic electron transport systems are the cytochromes, flavoproteins, iron-sulfur proteins, and the quinones.
Mar 27, 2009 The main function of cellular respiration is to break down molecules and generate ATP. Carbon dioxide is a product of aerobic respiration; lactic acid is a product of anaerobic respiration.
Cellular respiration is the process by which cells in plants and animals break down sugar and turn it into energy, which is then used to perform work at the cellular level. The purpose of cellular respiration is simple: it provides cells with the energy they need to function.
In aerobic respiration, the final electron acceptor (i.e., the one having the most positive redox potential) at the end of the ETS is an oxygen molecule (O2) that becomes reduced to water (H2O) by the final ETS carrier. This electron carrier, cytochrome oxidase, differs between bacterial types and can be used to differentiate closely related bacteria for diagnoses. For example, the gram-negative opportunist Pseudomonas aeruginosa and the gram-negative cholera-causing Vibrio cholerae use cytochrome c oxidase, which can be detected by the oxidase test, whereas other gram-negative Enterobacteriaceae, like E. coli, are negative for this test because they produce different cytochrome oxidase types.
There are many circumstances under which aerobic respiration is not possible, including any one or more of the following:
- The cell lacks genes encoding an appropriate cytochrome oxidase for transferring electrons to oxygen at the end of the electron transport system.
- The cell lacks genes encoding enzymes to minimize the severely damaging effects of dangerous oxygen radicals produced during aerobic respiration, such as hydrogen peroxide (H2O2) or superoxide [latex]left({text{O}}_{2}^{-}right)[/latex].
- The cell lacks a sufficient amount of oxygen to carry out aerobic respiration.
One possible alternative to aerobic respiration is anaerobic respiration, using an inorganic molecule other than oxygen as a final electron acceptor. Vietcong 2 cd key generator. There are many types of anaerobic respiration found in bacteria and archaea. Denitrifiers are important soil bacteria that use nitrate [latex]left(text{NO}_{3}^{-}right)[/latex] and nitrite [latex]left({text{NO}}_{2}^{-}right)[/latex] as final electron acceptors, producing nitrogen gas (N2). Many aerobically respiring bacteria, including E. coli, switch to using nitrate as a final electron acceptor and producing nitrite when oxygen levels have been depleted.
Microbes using anaerobic respiration commonly have an intact Krebs cycle, so these organisms can access the energy of the NADH and FADH2 molecules formed. However, anaerobic respirers use altered ETS carriers encoded by their genomes, including distinct complexes for electron transfer to their final electron acceptors. Smaller electrochemical gradients are generated from these electron transfer systems, so less ATP is formed through anaerobic respiration.
The Main Function Of Cellular Respiration Is To Generate
Think about It
- Do both aerobic respiration and anaerobic respiration use an electron transport chain?
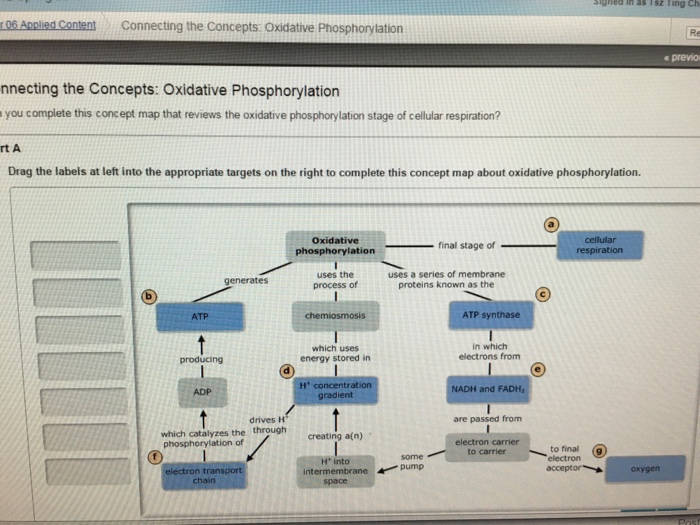
Chemiosmosis, Proton Motive Force, and Oxidative Phosphorylation
In each transfer of an electron through the ETS, the electron loses energy, but with some transfers, the energy is stored as potential energy by using it to pump hydrogen ions (H+) across a membrane. In prokaryotic cells, H+ is pumped to the outside of the cytoplasmic membrane (called the periplasmic space in gram-negative and gram-positive bacteria), and in eukaryotic cells, they are pumped from the mitochondrial matrix across the inner mitochondrial membrane into the intermembrane space. There is an uneven distribution of H+ across the membrane that establishes an electrochemical gradient because H+ ions are positively charged (electrical) and there is a higher concentration (chemical) on one side of the membrane. This electrochemical gradient formed by the accumulation of H+ (also known as a proton) on one side of the membrane compared with the other is referred to as the proton motive force (PMF). Because the ions involved are H+, a pH gradient is also established, with the side of the membrane having the higher concentration of H+ being more acidic. Beyond the use of the PMF to make ATP, as discussed in this chapter, the PMF can also be used to drive other energetically unfavorable processes, including nutrient transport and flagella rotation for motility.
Figure 1. ATP synthase is a complex integral membrane protein through which H+ flows down an electrochemical gradient, providing the energy for ATP production by oxidative phosphorylation. (credit: modification of work by Klaus Hoffmeier)
The potential energy of this electrochemical gradient generated by the ETS causes the H+ to diffuse across a membrane (the plasma membrane in prokaryotic cells and the inner membrane in mitochondria in eukaryotic cells). This flow of hydrogen ions across the membrane, called chemiosmosis, must occur through a channel in the membrane via a membrane-bound enzyme complex called ATP synthase (Figure 1). The tendency for movement in this way is much like water accumulated on one side of a dam, moving through the dam when opened. ATP synthase (like a combination of the intake and generator of a hydroelectric dam) is a complex protein that acts as a tiny generator, turning by the force of the H+ diffusing through the enzyme, down their electrochemical gradient from where there are many mutually repelling H+ to where there are fewer H+. In prokaryotic cells, H+ flows from the outside of the cytoplasmic membrane into the cytoplasm, whereas in eukaryotic mitochondria, H+ flows from the intermembrane space to the mitochondrial matrix. The turning of the parts of this molecular machine regenerates ATP from ADP and inorganic phosphate (Pi) by oxidative phosphorylation, a second mechanism for making ATP that harvests the potential energy stored within an electrochemical gradient.
The number of ATP molecules generated from the catabolism of glucose varies. For example, the number of hydrogen ions that the electron transport system complexes can pump through the membrane varies between different species of organisms. In aerobic respiration in mitochondria, the passage of electrons from one molecule of NADH generates enough proton motive force to make three ATP molecules by oxidative phosphorylation, whereas the passage of electrons from one molecule of FADH2 generates enough proton motive force to make only two ATP molecules. Thus, the 10 NADH molecules made per glucose during glycolysis, the transition reaction, and the Krebs cycle carry enough energy to make 30 ATP molecules, whereas the two FADH2 molecules made per glucose during these processes provide enough energy to make four ATP molecules. Overall, the theoretical maximum yield of ATP made during the complete aerobic respiration of glucose is 38 molecules, with four being made by substrate-level phosphorylation and 34 being made by oxidative phosphorylation (Table 1). In reality, the total ATP yield is usually less, ranging from one to 34 ATP molecules, depending on whether the cell is using aerobic respiration or anaerobic respiration; in eukaryotic cells, some energy is expended to transport intermediates from the cytoplasm into the mitochondria, affecting ATP yield.
Table 1 summarizes the theoretical maximum yields of ATP from various processes during the complete aerobic respiration of one glucose molecule.
| Table 1 | |||||
|---|---|---|---|---|---|
| Source | Carbon Flow | Molecules of Reduced Coenzymes Produced | Net ATP Molecules Made by Substrate-Level Phosphorylation | Net ATP Molecules Made by Oxidative Phosphorylation | Theoretical Maximum Yield of ATP Molecules |
| Glycolysis (EMP) | [latex]text{Glucose}left(6text{C}right)longrightarrow{2}text{ pyruvates}left(2text{C}right)[/latex] | 2 NADH | 2 ATP | 6 ATP from 2 NADH | 8 |
| Transition reaction | [latex]2text{ pyruvates}left(3text{C}right)longrightarrow{2}text{acetyl}left(2text{C}right)+2text{CO}_2[/latex] | 2 NADH | 6 ATP from 2 NADH | 6 | |
| Krebs cycle | [latex]2text{ acetyl}left(2text{C}right)longrightarrow{4}text{CO}_2[/latex] | 6 NADH 2 FADH2 | 2 ATP | 18 ATP from 6 NADH 4 ATP from 2 FADH2 | 24 |
| Total: | [latex]text{glucose}left(6text{C}right)longrightarrow{6}text{CO}_2[/latex] | 10 NADH 2 FADH2 | 4 ATP | 34 ATP | 38 ATP |
Think about It
- What are the functions of the proton motive force?
Key Concepts and Summary
- Most ATP generated during the cellular respiration of glucose is made by oxidative phosphorylation.
- An electron transport system (ETS) is composed of a series of membrane-associated protein complexes and associated mobile accessory electron carriers. The ETS is embedded in the cytoplasmic membrane of prokaryotes and the inner mitochondrial membrane of eukaryotes.
- Each ETS complex has a different redox potential, and electrons move from electron carriers with more negative redox potential to those with more positive redox potential.
- To carry out aerobic respiration, a cell requires oxygen as the final electron acceptor. A cell also needs a complete Krebs cycle, an appropriate cytochrome oxidase, and oxygen detoxification enzymes to prevent the harmful effects of oxygen radicals produced during aerobic respiration.
- Organisms performing anaerobic respiration use alternative electron transport system carriers for the ultimate transfer of electrons to the final non-oxygen electron acceptors.
- Microbes show great variation in the composition of their electron transport systems, which can be used for diagnostic purposes to help identify certain pathogens.
- As electrons are passed from NADH and FADH2 through an ETS, the electron loses energy. This energy is stored through the pumping of H+ across the membrane, generating a proton motive force.
- The energy of this proton motive force can be harnessed by allowing hydrogen ions to diffuse back through the membrane by chemiosmosis using ATP synthase. As hydrogen ions diffuse through down their electrochemical gradient, components of ATP synthase spin, making ATP from ADP and Pi by oxidative phosphorylation.
- Aerobic respiration forms more ATP (a maximum of 34 ATP molecules) during oxidative phosphorylation than does anaerobic respiration (between one and 32 ATP molecules).
Multiple Choice
Which is the location of electron transports systems in prokaryotes?
- the outer mitochondrial membrane
- the cytoplasm
- the inner mitochondrial membrane
- the cytoplasmic membrane
Which is the source of the energy used to make ATP by oxidative phosphorylation?
- oxygen
- high-energy phosphate bonds
- the proton motive force
- Pi
A cell might perform anaerobic respiration for which of the following reasons?
- It lacks glucose for degradation.
- It lacks the transition reaction to convert pyruvate to acetyl-CoA.
- It lacks Krebs cycle enzymes for processing acetyl-CoA to CO2.
- It lacks a cytochrome oxidase for passing electrons to oxygen.
In prokaryotes, which of the following is true?
- As electrons are transferred through an ETS, H+ is pumped out of the cell.
- As electrons are transferred through an ETS, H+ is pumped into the cell.
- As protons are transferred through an ETS, electrons are pumped out of the cell.
- As protons are transferred through an ETS, electrons are pumped into the cell.
Which of the following is not an electron carrier within an electron transport system?
- flavoprotein
- ATP synthase
- ubiquinone
- cytochrome oxidase
 Show Answer
Show AnswerFill in the Blank
The final ETS complex used in aerobic respiration that transfers energy-depleted electrons to oxygen to form H2O is called ________.
Show Answer
The passage of hydrogen ions through ________ down their electrochemical gradient harnesses the energy needed for ATP synthesis by oxidative phosphorylation.
Show AnswerA Key Function Of Cellular Respiration Is To Generate Quizlet
True/False
All organisms that use aerobic cellular respiration have cytochrome oxidase.
Think about It
Advantages Of Cellular Respiration
- What is the relationship between chemiosmosis and the proton motive force?
- How does oxidative phosphorylation differ from substrate-level phosphorylation?
- How does the location of ATP synthase differ between prokaryotes and eukaryotes? Where do protons accumulate as a result of the ETS in each cell type?
Cellular respiration is the process by which cells convert food energy like glucose into a form of energy that can be used to build and repair tissue and carry on other cell functions. Coenzyme A, synthesized by the body from pantothenic acid, or vitamin B-5, plays a key role in aerobic cellular respiration.
 Running your day-to-day Business Operations:- Set up all the required tax rates, and maintain GSTIN details of all your suppliers and customers- Ensure that all new transactions are GST-compliant, and print GST- invoices2. You can go to Form GSTR-1 report, apply corrections to vouchers that appear in incomplete/mismatch of information and export the same to MS excel template, which can then be validated using GSTN offline tool and uploaded to the GSTN portal- GST-ready Tally.ERP 9 allows to print Form GSTR 3B, which will generate a word document. How Installing Tally will Help in GST Compliance?With Tally.ERP 9 release 6.1, you can start running your entire business operations to comply with GST rules using the following capabilities:1. Submitting your Compliance Returns:- GST-ready Tally.ERP 9 allows to generate GSTR-1 from within the product itself.
Running your day-to-day Business Operations:- Set up all the required tax rates, and maintain GSTIN details of all your suppliers and customers- Ensure that all new transactions are GST-compliant, and print GST- invoices2. You can go to Form GSTR-1 report, apply corrections to vouchers that appear in incomplete/mismatch of information and export the same to MS excel template, which can then be validated using GSTN offline tool and uploaded to the GSTN portal- GST-ready Tally.ERP 9 allows to print Form GSTR 3B, which will generate a word document. How Installing Tally will Help in GST Compliance?With Tally.ERP 9 release 6.1, you can start running your entire business operations to comply with GST rules using the following capabilities:1. Submitting your Compliance Returns:- GST-ready Tally.ERP 9 allows to generate GSTR-1 from within the product itself.
Location Of Cellular Respiration
Glycolysis
Glycolysis is the first step in cellular respiration. It is the process by which cellular metabolism begins converting glucose, the main fuel used by the body obtained from starches and sugars, into usable energy. In glycolysis, glucose is partially oxidized, creating adenosine tri-phosphate, or ATP, the nucleotide that stores energy in the body in a form cells can readily use, according to the Johnson County Community College. Glycolysis also produces some waste in the form of carbon dioxide, which is exhaled, and an acetyl group called pyruvic acid, which then joins with coenzyme A for the next step of cellular respiration.
Acetyl Coenzyme A Formation
After glycolysis, pyruvic acid enters the cell mitochondrion, where it combines with coenzyme A to form acetyl CoA, according to the Clinton Community College. In the process, each pyruvic acid molecule loses one carbon atom, which combines with available oxygen to produce carbon dioxide, which is released through exhaling. Nicotinamide adenine dinucleotide, or NAD, also carries away hydrogen in the process of oxidation, becoming NADH. The remaining carbon atoms bond with coenzyme A, creating acetyl CoA.
Kreb's Cycle
When oxygen is present, cellular respiration continues after glycolysis with a process called the Kreb's cycle. In the Kreb's cycle, Acetyl CoA combines with a four-carbon compound in the mitochondria. Coenzyme A is released once again back into the cell structure, while the two carbons that had rendered it an acetyl group join the four-carbon compound, turning it into a six=carbon compound. This six-carbon compound combines with the oxygen from the NADH in a series of steps that generates more ATP, the chief storage structure of cellular energy.
Function Of Fermentation
Sources and Interactions
A Key Function Of Cellular Respiration Is To Generate: Pdf
Coenzyme A is created in the body from dietary components, most notably pantothenic acid, according to the Oregon State University Linus Pauling Institute. Pantothenic acid deficiency is rare, occurring only in cases of extreme malnutrition. Dietary sources of pantothenic acid include yogurt and milk, fish, chicken and eggs, lentils and peas, and yeast breads. Oral contraceptives might increase the need for pantothenic acid intake. Taking pantethine, a version of pantothenic acid used to lower cholesterol, along with statins might enhance the statins' effect on serum lipids.